this post was submitted on 21 Oct 2024
1273 points (98.1% liked)
Microblog Memes
5787 readers
2899 users here now
A place to share screenshots of Microblog posts, whether from Mastodon, tumblr, ~~Twitter~~ X, KBin, Threads or elsewhere.
Created as an evolution of White People Twitter and other tweet-capture subreddits.
Rules:
- Please put at least one word relevant to the post in the post title.
- Be nice.
- No advertising, brand promotion or guerilla marketing.
- Posters are encouraged to link to the toot or tweet etc in the description of posts.
Related communities:
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
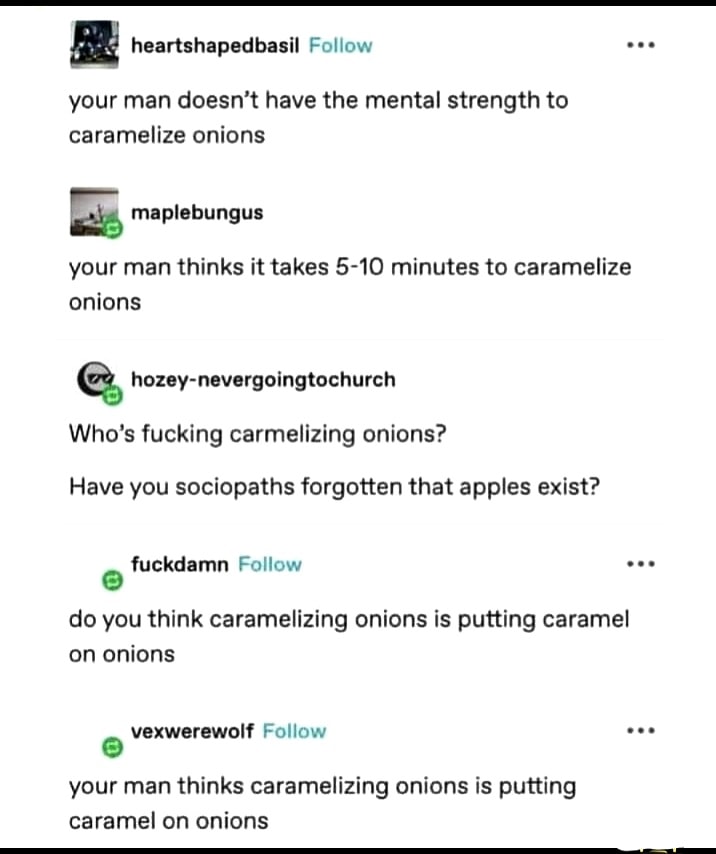
Heating a solution of aqueous bicarbonate will release carbon dioxide, too. But since we have delicious onions and stuff in there too, let's walk through my thought process: Baking powder is baking soda + weak acid + cornstarch (to prevent premature reaction). Since the speed-up for the Maillard reaction works by deprotonating amino groups to make them more nucleophilic, the acid-base reaction that releases CO~2~ when using baking powder will still occur with just baking soda + food (ie: the protiens in the food are acting as the acid). You're probably right that using baking powder would produce more CO~2~, or at least produce it faster, but reducing carcinogenic side products for Maillard reactions via CO~2~ is a low-priority concern for me anyway. Just a fun curiousity that occured to me when reading the wiki page!
Sorry if my carbon dioxide subscripts don't work. I don't think my client supports all the fancy markdown, but i tried my best.