this post was submitted on 29 Oct 2024
497 points (99.4% liked)
Science Memes
11441 readers
947 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
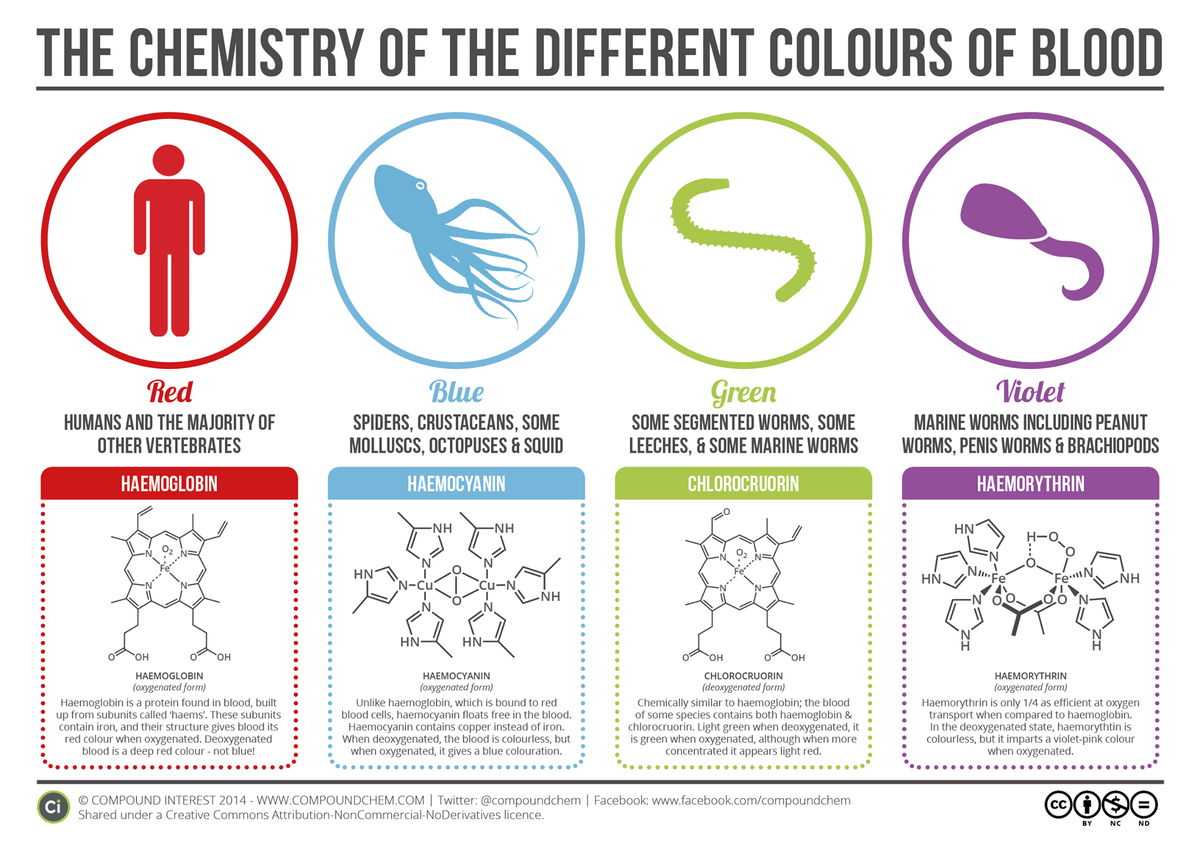
Yeah, I didn't do the carbonic acid, then there's the increased bicarb buffering around the pleura, couple other facts.
https://www.ncbi.nlm.nih.gov/books/NBK539815/
Thus the acidity causes o2 release. Temperature (lungs tend to be very cold in the body) is important too.
It's amazing how subtly it works to gently increase efficiency where we need it. Otherwise it's just a very weak oxygen bond (which is hard enough given oxygen is extremely non-polar and all you have are the valence pairs. edit: This lead me to wonder how the fuck it even bonded effectively
https://www.jbc.org/article/S0021-9258(19)63845-7/fulltext
Wow, I'm impressed, they're using spin-coupling which is a pretty dicey effect.
That's some fucking crazy ass engineering by nature, A weak, highly reversible bond with the molecule keyed to both pH and thermal triggers. That was a fun rabbit hole.
Thanks so much for the deep dive. I love learning from such concise facts.
To add, also @[email protected] : There is only one known species of vertebrates without hemoglobin. The Crocodile Icefish, it once had it in its blood and lost some genes to synthesize it. The debate about why is still ongoing, with the currently favored theory that they adapted to a high oxygen and low iron environment.
Thanks for the callout and link! 🙏🏽
Nature is fascinating.